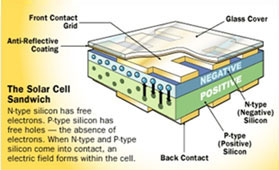
Placing electrical contacts :
Electrical contacts connect each solar cell to another and to the receiver of produced
current. The contacts must be very thin (at least in the front) so as not to block
sunlight to the cell. Metals such as palladium/silver, nickel, or copper are vacuum
evaporated through photoresist, silkscreened, or merely deposited on the exposed
portion of cells that have been partially covered with wax. All three methods involve
a system in which the part of the cell on which a contact is not desired is protected,
while the rest of the cell is exposed to the metal. After the contacts are in place,
thin strips ("fingers") are placed between cells.
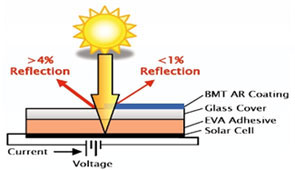
Anti-reflective coating :
The most commonly used strips are tin-coated copper. This is because pure silicon
is shiny, it can reflect up to 35 percent of the sunlight. To reduce the amount
of sunlight lost, an anti-reflective coating is put on the silicon wafer. The most
commonly used coatings are titanium dioxide and silicon oxide, though others are
used. The material used for coating is either heated until its molecules boil off
and travel to the silicon and condense, or the material undergoes sputtering. In
this process, a high voltage knocks molecules off the material and deposits them
onto the silicon at the opposite electrode. Yet another method is to allow the silicon
itself to react with oxygen- or nitrogen-containing gases to form silicon dioxide
or silicon nitride. Commercial solar cell manufacturers use silicon nitride.